Fancy diamonds get their unique shades in a variety of ways.
The Spectrum of Light
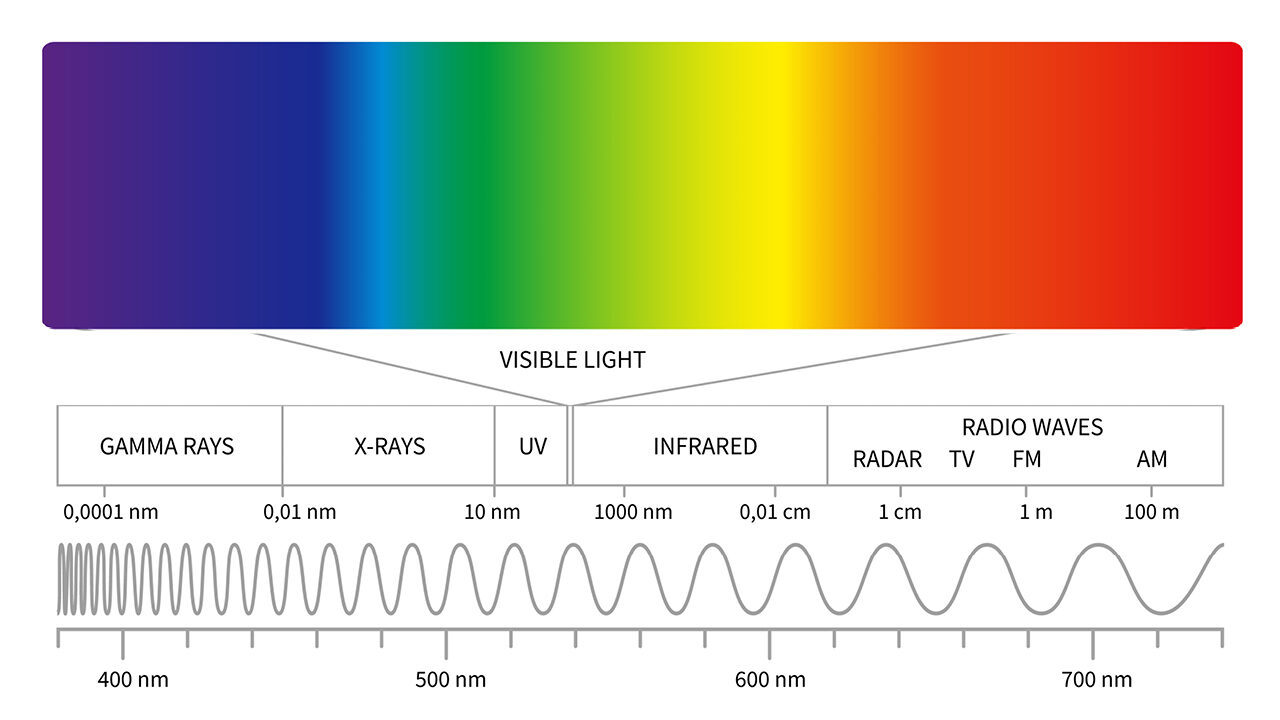
Very much like sound, light travels in waves. It is an electromagnetic radiation (like radio waves or X-rays) that is visible to the human eye. A single wavelength produces a monochromatic ray of light and is analogous to playing a single note on an instrument, but most light we see is polychromatic: Like a musical chord or a complex sound, it combines many different wavelengths.
This collection of wavelengths and their intensities is called the spectrum of the light. When a ray of light enters some transparent medium at a sharp angle, the ray of light bends. This is how eyeglasses can correct your vision or how people look shorter in a swimming pool.
More importantly for us, this is how polishers bend light to make diamonds shine! And for color lovers, another fascinating phenomenon occurs: different wavelengths bend by different amounts, and so as a ray of complex polychromatic light is bent by a crystal, we can see it being decomposed into the different wavelengths that make up its spectrum. This is what makes rainbows appear on a rainy sunny day.
This is also what creates fire in a diamond, these little sparks of different colors.
Seeing Color
Unfortunately, our human eyes are extremely limited, and without the help of a crystal we can only perceive very small chunks of the spectrum. Our eyes are equipped with just three types of receptors (cones) that can sense light in three ranges of wavelengths. We see those as levels of red, green and blue.
Our brain then recombines these three signals to construct a color sensation, and associates these combined color sensations with natural items in everyday life. This is why saying the words “Banana” or “Tangerine” awakens those colors in our minds, yet few would think of them as being just a mix of red and green.
White light is any light that has red, green and blue wavelengths in similar proportions as natural light (from the sun). So artificial white light (from a light bulb or a diamond grading lamp) appears very similar to sunlight while having a very different spectrum, yet some objects (especially natural color diamonds) can appear to have radically different colors under different lights.
The way we perceive colors depends on many other factors, such as the position of the object, surrounding colors, or even our mood.
What Gives Diamonds Color?
As rays of white light enter a diamond, its material absorbs some of the wavelengths of the spectrum while allowing others to escape.
For example, nitrogen atoms in type I diamonds cause them to absorb blue wavelengths letting
out a yellow, orange or brown color.
The presence of boron in the diamond’s lattice can create a blue shade. Twists and distortions in a diamond lattice can give it a yellow, brown or even pink and red color.
The color in green diamonds is caused by exposure from radioactive rocks near the Earth’s surface. The radiation knocks out some carbon atoms from their position in the diamond
lattice, and this causes the diamond to absorb red wavelengths.
Only one in 10,000 diamonds has a natural color, and each color occurs as a result of extremely rare circumstances.
Natural color diamond experts can often, just by observing the diamond, retrace part of their unique history and their provenance.
Image: Shutterstock